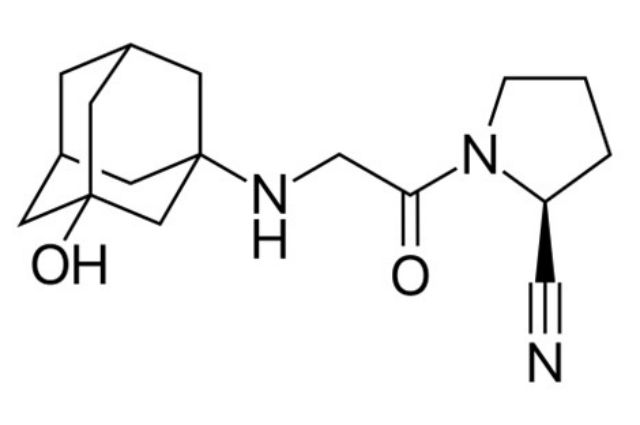
Structural diagram of vildagliptin - Sold under trade names Galvus and Zomelis. Created using ACD/ChemSketch 8.0 and Inkscape.
Image by Fvasconcellos - Wikipedia
Introduction:
Vildagliptin has a melting point of roughly 1500 C and is a white to slightly yellowish or greyish crystalline powder. It has a chemical formula of 1 and is easily soluble in water. [(3-hydroxy adamant-1-arylamino) acetyl]- Pyrrolidine-2(S)-carbonitrile, has a molecular weight of 303.40 and a formula of C17H25N3O2.
Vildagliptin is a widely used DPP4 Blocking agent which is used to treat type 2 diabetes.
Pharmacology:
- Pharmacodynamics:
Vildagliptin is a high-affinity dipeptidyl-peptidase-4 (DPP4) inhibitor that stimulates islet cell insulin secretion via an enhanced incretin action. It also improves glycemic control.
Vildagliptin injection causes a quick and nearly complete suppression of DPP-4 activity. In comparison to DPP-4, vildagliptin exhibits poor inhibition of and quick dissociation from DPP-8 and DPP-9. Vildagliptin treatment caused the DPP-4 enzyme activity to be inhibited for 24 hours in patients with type 2 diabetes. Increased fasting and postprandial endogenous levels of the incretin hormones GLP-1 (glucagon-like peptidase-1) and GIP (glucose-dependent insulinotropic polypeptide) are brought on by vildagliptin's suppression of DPP-4.
Vildagliptin does not increase insulin secretion or lower blood sugar levels in non-diabetic people, therefore the degree of beta-cell function improvement depends on the starting amount of impairment.
Vildagliptin improves the sensitivity of alpha cells to glucose and decreases glucagon production by raising endogenous GLP-1 levels. Inappropriate glucagon release during meals is decreased. Thus, it is possible to anticipate that the increase in incretin hormone levels associated with hyperglycemia may limit postprandial hepatic glucose synthesis, resulting in lower glycemia.
Vildagliptin therapy does not produce the expected delay in stomach emptying caused by elevated GLP-1 levels.
Pharmacokinetics:
- Linearity:
Vildagliptin has a 58% absolute oral bioavailability and is quickly absorbed. Over the therapeutic dose range, vildagliptin's peak plasma concentration and area under the plasma concentration versus time curve (AUC) both rose in a roughly dose-proportional manner.
- Absorption:
Vildagliptin is quickly absorbed after oral treatment during fasting, with peak plasma concentrations being noted after 1.75 hours. Vildagliptin's rate of absorption is marginally slowed by co-administration with food, as evidenced by a 19% reduction in peak plasma concentrations and a 2.5-hour delay before peak plasma concentration. Both the extent of absorption and the total exposure (AUC) are unaffected by meal.
- Distribution:
Vildagliptin has a low (9.3%) plasma protein binding affinity and is distributed equally between red blood cells and plasma. Vildagliptin's mean steady-state distribution volume following intravenous treatment is L, indicating extravascular distribution.
- Metabolism:
Human vildagliptin is primarily eliminated by metabolism, which accounts for 69% of the dosage. The cyano moiety's hydrolysis product, LAY151, which makes up 57% of the dose and is pharmacologically inactive, is the primary metabolite. The amide hydrolysis product makes up 4% of the dose. According to an in vitro experiment using DPP-4-impaired rats, DPP-4 plays a small but significant role in the hydrolysis of vildagliptin. In vitro experiments showed that vildagliptin does not inhibit or stimulate cytochrome P450 enzymes, and that vildagliptin is not quantifiably metabolized by cytochrome P450 enzyme.
Excretion and Elimination:
Following oral administration, vildagliptin is eliminated in the urine in about 85% of the dose and is recovered in the feces in 15% of the dose. After oral treatment, 23% of the dose of vildagliptin is excreted unaltered through the kidneys. Vildagliptin's total plasma and renal clearances following IV treatment in healthy people are 14L/hour and 13L/hour, respectively. After IV administration, the typical elimination half-life is around two hours. After oral administration, the half-life of elimination is approximately 3 hours and is dose-independent.
Special Populations:
- Age:
When compared to younger, healthy patients (18–40 years), the overall exposure to vildagliptin was raised in older subjects (70 years) by 32%, with an increase in peak plasma concentration of 18%. The clinical relevance of these modifications is not thought to exist. In the age groups examined, vildagliptin's ability to inhibit DPP-4 is not influenced by age.
- Gender:
The pharmacokinetics of vildagliptin did not change between male and female individuals, regardless of age or BMI. Vildagliptin's ability to inhibit DPP-4 was not influenced by gender.
- Pediatric:
No pharmacokinetic data were available.
- Obesity:
The pharmacokinetic characteristics of vildagliptin are not affected by BMI. BMI had no impact on vildagliptin's ability to inhibit DPP-4.
Hepatic Impairment:
In comparison to participants with normal hepatic function, subjects with mild, moderate, and severe hepatic impairment were investigated to see how impaired hepatic function affected the pharmacokinetics of vildagliptin. In participants with mild and severe hepatic impairment, the exposure to vildagliptin after a single dosage was raised by 22%. The highest change in vildagliptin exposure is only 30%, which is not regarded as clinically relevant. The degree of hepatic function impairment was not correlated with variations in vildagliptin exposure.
Vildagliptin should not be used in patients with hepatic impairment, particularly those whose pre-treatment ALT or AST levels were higher than 2.5 times the upper limit of normal.
Chronic Kidney Disease:
In comparison to subjects with normal renal function, systemic exposure to vildagliptin was higher in subjects with mild, moderate, and severe chronic illness as well as patients with end-stage renal disease who were receiving hemodialysis. As chronic renal disease became more severe, there was an increase in exposure to the inactive metabolite. While exposure to the inactive metabolite correlated with the severity of chronic kidney disease, increases in vildagliptin exposure did not. The chronic renal disease does not affect the elimination half-life of vildagliptin. Patients with ESRD receiving hemodialysis or those with moderate to severe chronic renal disease do not need to change their dosage.
Race:
There is no evidence that race affects the pharmacokinetics of Vildagliptin.
Clinical Trials:
Over 15,000 people with type 2 diabetes took part in double-blind, placebo- or active-controlled research trials, some of which lasted longer than two years. More than 9000 individuals received daily dosages of 50 mg once daily, 50 mg twice daily, or 100 mg once daily vildagliptin in these investigations. Vildagliptin was administered once daily to about 5000 male and 4000 female patients who were under 65 years old.
Vildagliptin was used in these trials as a monotherapy for type 2 diabetes patients who were drug-naive or in conjunction with other medications for patients whose diabetes was not sufficiently managed. According to studies on monotherapy, vildagliptin was marginally less effective when taken alone than sulfonylureas or pioglitazone. There are no data on morbidity or mortality.
Overall, vildagliptin improved glycemic control whether it was administered alone or with metformin, as determined by clinically meaningful drops in HbA1c from baseline at the study's endpoint.
Patients with higher baseline HbA1c levels experienced greater HbA1c reductions with vildagliptin in clinical trials.
Table 1: Key efficacy results of vildagliptin in placebo-controlled monotherapy trials
Monotherapy | Primary endpoint (weeks) | Mean baseline HbA1c | Mean change from baseline in HbA1c (%) | Difference from the placebo group (95%Cl) | Patients achieving a ≥ 0.7% reduction in A1c% |
Vildagliptin 50 mg once daily(N=104) [study23 1] | 24 | 8.2 | -0.8 | -0.5** (-0.8, -0.1) | 62 (60%) |
Vildagliptin 50mg twice daily(N=90) [study23 1] | 24 | 8.6 | -0.8 | -0.5** (-0.8, -0.1) | 59 (66%) |
| Vildagliptin 50 mg once daily (N=84) [study 23 4] | 24 | 8.3 | -0.5 | -0.5** (-0.9, -0.1) | 37 (44%) |
| Vildagliptin 50mg once daily (N=79) [study23 4] | 24 | 8.4 | -0.7 | -0.7** (-1.1, -0.4) | 43 (54%) |
**p< 0.05 for comparison vs placebo
Indications:
Vildagliptin 50 is indicated as an adjunct to diet and exercise to improve glycemic control in persons 18 years of age and older with type 2 diabetes mellitus.
Interactions with other medicines:
There is little chance for medication interactions with Vildagliptin 50. Vildagliptin is unlikely to interact with concurrent drugs that are substrates, inhibitors, or inducers of cytochrome (CYP) P450 enzymes because it does not inhibit or induce CYP P450 enzymes.
Vildagliptin is also unlikely to interact with concurrent drugs that are substrates, inhibitors, or inducers of CYP P450 enzymes. It also has no known effects on the metabolic clearance of co-medications metabolized by CYP 1A2, CYP 2C8, CYP 2C9, CYP 2C19, CYP 2D6, CYP 2E1, and CYP 3A4/5.
Adverse Effects:
3,784 individuals who participated in controlled trials lasting at least 12 weeks and were given vildagliptin at doses of 50 mg once a day or 100 mg (50 mg BD), once daily, were the source of the safety data. Vildagliptin was administered to 2,264 of these patients as monotherapy and 1,520 of them as part of a combination therapy with another medication. Vildagliptin was administered to 2,682 individuals daily and 1102 patients on a one-day basis.
In these trials, the majority of ADRs were minor, temporary discontinuations. There was no correlation between adverse reactions and age, gender, ethnicity, exposure time, or daily dose.
Vildagliptin has a similar rate of reported rare instances of angioedema as controls. Vildagliptin was provided in conjunction with an ACE inhibitor, which resulted in a higher percentage of instances being reported. Most of the incidents had a low severity and went away with continued vildagliptin therapy.
There have been a few isolated reports of hepatic malfunction, including hepatitis. These patients were often asymptomatic, had no clinical consequences, and after treatment was stopped, liver function tests returned to normal. The incidence of ALT or AST increases >=3xULN was 0.2%, 0.3%, and 0.2% for vildagliptin 50mg daily, vildagliptin 50mg BD, and all comparators, respectively, according to data from control monotherapy and add-on therapy trials lasting up to 24 weeks. These elevations in transaminases were generally asymptomatic, nonprogressive in nature, and not associated with cholestasis or jaundice.
Below, for each indication, is a list of adverse events observed in patients who received vildagliptin as monotherapy and add-on medication in double-blind studies, organized by system organ class and absolute frequency. The terms very common (1/10) and common (1/100, 1/10) are used to describe frequencies. ADRs are listed inside each frequency grouping in decreasing order of seriousness.
Monotherapy:
In general, patients treated with vildagliptin at a dose of 50 mg once daily or vildagliptin at a dose of 50 mg BD were not more likely to discontinue from monotherapy trials due to adverse events than patients treated with placebo, who served as comparators. Hypoglycemia was uncommon in monotherapy studies, occurring in 0.5% of patients treated with vildagliptin 50mg once daily and 0.3% of patients treated with vildagliptin 50mg BD, compared to 0.2% of patients in the groups treated with an active comparator or placebo, with no severe events reported.
Vildagliptin is administered as monotherapy in neutral weights.
Adverse Drug Reactions:
Nervous System Disorders:
- Common – Dizziness
- Un-common – Headache
Gastrointestinal Disorders:
- Un-common – Constipation
General Disorders and Administration Site Conditions:
- Un-common – Oedema peripheral
- Vildagliptin monotherapy did not demonstrate any extra safety signals or unexpected concerns in long-term clinical trials lasting up to 2 years.
Dosage and administration:
- A 50 mg or 100 mg dosage is typically taken once daily, divided into two 50 mg doses.
- Vildagliptin is not advised for use in children.
- Doses beyond 100mg are not suggested.
- You can take vildagliptin orally with or without food.
Storage:
Stored, secure, and maintained at a temperature of no more than 30 o C.
