Executive Summary
The development of 'Bone 02', a novel medical adhesive unveiled by a Chinese research team, represents a significant, potentially paradigm-shifting advancement in the long-sought field of bio-inspired bone adhesives. The material's reported properties directly address major, long-standing limitations of conventional fracture fixation methods. The claims of rapid, in-situ fracture fixation in a blood-rich environment and natural bioabsorption, which eliminates the need for a second surgery to remove implants, position 'Bone 02' as a potential revolutionary technology.
Based on media reports citing a clinical study on over 150 patients, 'Bone 02' offers a minimally invasive alternative that drastically reduces surgical time, lowers infection risks, and improves patient outcomes. Its reported mechanical properties, specifically its shear and compressive strengths, appear to meet or exceed minimum clinical requirements for certain applications.
However, a fundamental caveat in the available information is the reliance on news reports from local and international media outlets. A confirmed, peer-reviewed scientific publication or clinical trial registry data for 'Bone 02' has not been identified in the provided materials. This information gap is crucial for a professional audience, as it prevents the independent verification of the bold claims and detailed analysis of the adhesive's precise mechanism, long-term safety, and efficacy.
While currently presented as a breakthrough in a pre-publication format, 'Bone 02' illustrates the accelerating trend in biomaterials toward intelligent, functional hydrogels and bio-inspired composites. Its successful transition from a local news story to a globally adopted medical device will ultimately hinge on transparent scientific validation and navigating stringent regulatory pathways. The innovation, regardless of its immediate clinical availability, serves as a powerful example of the future direction of orthopedic biomaterials.
The Persistent Challenge of Fracture Fixation
The Burden of Musculoskeletal Injuries
Bone is a remarkable and dynamic biomaterial, uniquely capable of self-healing and regeneration in response to the physiological and mechanical demands placed upon it. This continuous process of bone modeling and remodeling, driven by osteoblasts that deposit new bone matrix and osteoclasts that resorb old material, allows the skeleton to adapt and maintain its structural integrity throughout a person's life. Despite this innate resilience, bone fractures remain a significant and widespread global health concern. Comminuted fractures—where the bone shatters into multiple fragments—are particularly challenging to treat, affecting tens of millions of people each year and imposing a substantial burden on healthcare systems.
For nearly a century, the quest for a true bone adhesive has been viewed as a "holy grail" in orthopedic medicine. The persistent challenge has been to develop a material that can not only provide a strong, immediate bond to bone but can also function effectively within the human body's wet, blood-rich environment. Early attempts at developing such adhesives in the 1940s, which were based on materials like gelatin, epoxy resins, and acrylates, were ultimately abandoned due to fundamental issues with biocompatibility. This historical context is essential for understanding why a claimed breakthrough today holds such profound significance.
A History of Orthopedic Fixation
Current standard-of-care for fracture fixation, particularly for complex breaks, relies heavily on internal metal hardware, such as plates, screws, and rods. This approach, while effective, is associated with a number of well-documented limitations. The procedures are often time-consuming, requiring surgeons to spend hours meticulously piecing together shattered bone fragments. In cases of comminuted fractures, small bone fragments may be lost or discarded, leading to bone mass loss, delayed healing, or even non-union. For fractures near joints, imprecise repositioning can lead to long-term complications like arthritis, which severely impacts a patient's quality of life.
Furthermore, the significant difference in mechanical properties between rigid metal implants and natural bone tissue can lead to a complication known as stress shielding. The stiff metal hardware bears the majority of the mechanical load, which prevents the underlying bone from experiencing the stress it needs to stimulate natural healing and remodeling. Over time, this can cause the bone to weaken and even increase the risk of refracture after the implant is removed.
The Unmet Need for True Bone Adhesives
The orthopedic community has long recognized the need for a solution that avoids the drawbacks of metal fixation. Existing alternatives like bone cements and bone void fillers have been widely used, but they do not function as true adhesives. The most common bone cement, polymethyl methacrylate (PMMA), works by mechanically interlocking with the porous bone structure rather than forming a chemical bond. Its clinical use is also limited by a number of issues. The chemical reaction that causes PMMA to harden is highly exothermic, with temperatures reaching up to 56 degrees Celsius, which can cause significant tissue necrosis and local damage to surrounding bone. The material is also non-resorbable and releases potentially toxic monomers. While some biodegradable cements exist, they often suffer from poor mechanical strength and an inability to be completely replaced by new bone.
Other attempts at creating synthetic adhesives, such as cyanoacrylates, have also been met with challenges. These materials, while effective for skin closure, have shown limitations in wet environments and can be associated with inflammatory markers, restricting their use in load-bearing internal applications. The failure of these past efforts underscores the core challenge that has persisted for decades: developing a material that is not only strong and fast-acting but also biocompatible and able to form a robust, long-lasting bond directly to living bone tissue in a biologically active environment.
"Bone 02": A New Contender
Origins and Foundational Principles
The development of 'Bone 02' is reported to be the culmination of a multi-year research effort led by Dr. Lin Xianfeng, an associate chief orthopedic surgeon at Sir Run Run Shaw Hospital in Zhejiang Province, China. The inspiration for the adhesive's design came from an observation of oysters firmly clinging to a bridge underwater. This insight into how a biological organism could achieve powerful adhesion in a submerged, dynamic environment sparked the idea of creating a similar adhesive for the human body's blood-rich and moist internal environment.
This bio-inspired approach is a notable trend in modern biomaterials research. While the oyster is a unique muse for 'Bone 02', other concurrent research has been inspired by marine creatures such as the sandcastle worm, which secretes a natural adhesive to build its protective home. These parallel efforts, drawing from different natural models, demonstrate a global scientific consensus on the value of looking to nature for solutions to long-standing engineering and medical problems. Following this inspiration, Dr. Lin's team reportedly tested over 50 formulations and conducted hundreds of trials and animal experiments over several years before settling on the final product.
Technical Specifications and Performance Metrics
The most widely publicized claims for 'Bone 02' revolve around its exceptional speed and mechanical performance. The adhesive is reported to achieve precise fixation of bone fragments within two to three minutes, even in a blood-rich environment. This rapid curing time is a key advantage over traditional methods, which can require hours of meticulous surgical work.
The reported mechanical properties of 'Bone 02' provide a more specific, quantitative measure of its performance. The glued bones demonstrated a maximum bonding force of over 400 pounds, a shear strength of approximately 0.5 MPa, and a compressive strength of around 10 MPa. To understand the significance of these numbers, it is essential to compare them with established clinical and material benchmarks.
Table 1: Reported Mechanical Properties of 'Bone 02' vs. Industry Benchmarks
Metric | 'Bone 02' Value | Industry Benchmarks | ||
Shear Strength | ~0.5 MPa | Minimum Clinical Requirement for Adhesives: >0.2 MPa | Cancellous Bone Range: 0.5 to 1.0 MPa | Cortical Bone Range: 3 to 9 MPa |
Compressive Strength | 10 MPa | Non-Adhesive Hydrogel (HT-MPC): 26.8±9.5 MPa | Poly(methyl methacrylate) (PMMA): ≈70−100 MPa |
The reported shear strength of 0.5 MPa for 'Bone 02' is at the lower end of the desired clinical range for cancellous bone, which is the soft, porous bone found at the ends of long bones and in vertebrae. This value is above the minimum suggested clinical requirement of 0.2 MPa,
indicating its potential for use in low-load-bearing applications, such as fixing osteochondral fragments or repairing certain comminuted fractures. However, it falls well below the 3 to 9 MPa required for bonding to the denser, stronger cortical bone. This suggests that 'Bone 02', as currently described, may be better suited as an augmentation or stabilizing agent rather than as a primary load-bearing fixation for major long-bone fractures, which is a critical point of analysis not fully addressed in the provided news reports.
The compressive strength of approximately 10 MPa is within a clinically relevant range for certain applications, although other hydrogel formulations have demonstrated significantly higher compressive strengths. This suggests that the material's mechanical properties, while promising for a biological adhesive, may not be uniform across all types of orthopedic applications and would require further detailed testing.
Mechanism and Composition
Based on the available media reports, the precise chemical composition of 'Bone 02' is not disclosed. However, the described properties—an injectable material that cures quickly in a wet environment and is bioabsorbable—suggest a mechanism similar to other advanced biomaterials. For instance, a structurally and functionally dual-biomimetic bone adhesive described in other research is developed as an organic-inorganic network-enhanced hydrogel, integrating materials like caffeic acid-grafted collagen, aminated laponite, and polyethylene glycol. Another successful approach, inspired by the sandcastle worm, uses a mineral-organic composite of tetracalcium phosphate and phosphoserine.
Given the reported behavior of 'Bone 02', it is highly probable that its formulation employs a similar organic-inorganic hybrid strategy. The organic components would provide the crucial adhesive properties in the wet biological environment, while the inorganic components would offer structural reinforcement and act as a scaffold for new bone to grow into. The bioabsorbable nature of 'Bone 02', which is reportedly absorbed over six months, is consistent with this type of composite, where the scaffold slowly degrades and is replaced by native bone tissue. This approach is in direct contrast to non-degradable materials like PMMA and metal implants.
A Critical Review of Clinical Efficacy and Safety
Initial Clinical Findings
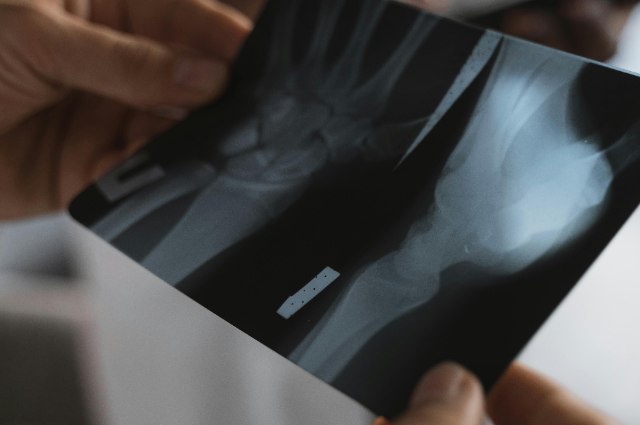
The most compelling information for 'Bone 02' comes from the claim that a "world's first clinical study" has been completed. This study, conducted on "over 150 patients" at Sir Run Run Shaw Hospital, reportedly demonstrated "full compliance in both safety and efficacy". One specific case mentioned in the reports details a young worker with a shattered wrist who received the treatment. Surgeons made a minimally invasive incision and fixed the fragments in three minutes. A review three months later showed excellent healing and a full restoration of wrist function, avoiding the trauma of traditional open surgery and the need for a secondary operation.
However, a fundamental and critical point of analysis for an expert audience is the absence of a primary, peer-reviewed scientific publication detailing these clinical results. The claims are exclusively sourced from local media reports and news agencies. There is no publicly available clinical trial registration number, which is a standard requirement for human trials in most major regulatory jurisdictions. This lack of independent, verifiable data is a significant red flag. The cornerstone of the scientific method is the ability for results to be replicated and validated by other researchers. Without access to the study's methodology, patient demographics, detailed outcomes, and long-term follow-up data, the global scientific community is unable to verify these groundbreaking claims.
Patient-Centric Outcomes
The reported benefits of 'Bone 02' extend far beyond the operating room, offering a comprehensive improvement to the patient experience. The minimally invasive procedure, performed through a small, 2 to 3 cm incision, dramatically reduces the trauma and blood loss associated with traditional open surgeries. The most transformative benefit for patients is the elimination of a second surgery to remove metal implants. This is a profound advantage that reduces the patient's pain, the risk of a second infection, and the financial and emotional burden of a repeat surgical procedure and recovery.
The patient case study involving the shattered wrist provides a tangible example of these benefits, highlighting a full recovery and restoration of function without the need for traditional hardware. This suggests a more rapid return to a normal quality of life, which is a major objective of modern orthopedic care.
The "Glue Gun" and Other Innovations
The 'Bone 02' adhesive is not the only innovation of its kind. A separate and concurrent research effort from South Korea has developed a novel "glue gun" device that 3D-prints bone grafts directly onto fractures during surgery. This technology also aims to reduce operative time and improve procedural efficiency by allowing surgeons to create custom, anatomically matched implants in real-time without the need for a long pre-operative planning and manufacturing process. The filament used in this device is composed of hydroxyapatite (HA), a natural bone component, and polycaprolactone (PCL), a biocompatible thermoplastic.
The "glue gun" innovation shares the core goals of 'Bone 02': speed, customization, and improved patient outcomes. The existence of these parallel projects from different parts of the world demonstrates that the field of orthopedic biomaterials is experiencing a renaissance, with Researchers globally are converging on new approaches to address the same fundamental challenges of fracture fixation.
Comparative Analysis: "Bone 02" in the Orthopedic Landscape
Versus Traditional Metal Fixation
The introduction of 'Bone 02' presents a compelling alternative to traditional metal fixation. The core differences lie not just in speed, but in fundamental mechanics and long-term biological outcomes.
Table 2: Comparative Analysis of Fracture Fixation Methods
Feature | Bone 02' (Injectable Glue) | Traditional Metal Fixation (Plates/Screws) | Traditional Bone Cements (PMMA, CPCs) |
Adhesiveness | Strong, wet-field adhesion | None | None; mechanical interlock only |
Surgical Time | Minutes (2-3 min) | Hours | Varies, but can be rapid |
Invasiveness | Minimally invasive (injection, small incision) | Highly invasive (large incision) | Varies (e.g., injection for vertebroplasty) |
Bioabsorbabilit-y | Yes, absorbed within ∼6 months | No | No/Limited (PMMA is not absorbable) |
Need for Second Surgery | No | Often required for implant removal | Not for removal, but revision is possible |
Key Limitations | Unverified claims, mechanical strength may be limited for high-load applications | Invasiveness, stress shielding, infection risk | Exothermic reaction, toxicity, poor mechanical properties, not true adhesives |
As Table 2 illustrates, 'Bone 02' offers a compelling clinical profile. The reduction in surgical time from hours to minutes would dramatically improve procedural efficiency. The ability to apply the adhesive through a minimally invasive incision would also reduce patient trauma, blood loss, and recovery time.
Most importantly, 'Bone 02' bypasses the problem of stress shielding. Unlike a rigid metal plate that prevents natural stress on the bone, 'Bone 02' is designed to gradually degrade over a period of approximately six months and be replaced by new, natural bone. This allows the body to remodel and heal the fracture with its own tissue, which is a more physiologically sound approach to bone repair. This biological advantage is the primary reason why bioabsorbable adhesives are considered a major step forward from conventional metal fixation.
Versus Bone Cements and Void Fillers
A key distinction must be made between 'Bone 02' and traditional bone cements. As multiple sources clarify, existing cements like PMMA are not adhesives and cannot form a chemical bond to bone tissue. They function primarily as fillers, locking into place through mechanical interdigitation, which can be insufficient for complex fractures and is prone to loosening over time. The exothermic reaction of PMMA also poses a direct risk of tissue damage and local complications.
In contrast, a review of other bio-inspired adhesives highlights their remarkable adhesive strength. A sandcastle worm-inspired material was found to be 10 times more adhesive than a bioresorbable calcium phosphate cement and 7.5 times more adhesive than non-resorbable PMMA. While the exact composition of 'Bone 02' is unknown, its claimed performance in a blood-rich environment and its adhesive properties suggest it belongs to this new class of true adhesives, offering a far more robust fixation than traditional cements.
Comparison with other Bio-inspired Adhesives
The development of 'Bone 02' is part of a broader, global trend in biomaterials research. For example, a bone adhesive inspired by the sandcastle worm, known as Tetranite™, is composed of tetracalcium phosphate and phosphoserine and is reportedly undergoing FDA approval. Another adhesive, inspired by mussels, integrates poly(vinyl alcohol) and L-DOPA amino acid to achieve strong adhesion.
While each of these materials draws from a different source of biological inspiration and may use slightly different chemical components, they all share the same fundamental objective: creating a material that combines robust adhesion in a wet environment with biocompatibility and bioabsorbability. The 'Bone 02' project, with its focus on an injectable, oyster-inspired formulation, represents a unique and promising contribution to this burgeoning field.
The Scientific and Regulatory Pathway Ahead
The Missing Piece: Peer-Reviewed Data
For a technology with such transformative clinical claims, the absence of a confirmed, peer-reviewed publication is the most significant hurdle to its credibility within the global scientific and medical communities. The provided materials repeatedly rely on local media reports from outlets like Zhejiang Online and Global Times. While these reports are a valuable public announcement, they do not constitute scientific evidence.
The peer-review process, a cornerstone of modern science, involves independent experts critically evaluating a research paper's methodology, data, and conclusions. This scrutiny is essential to validate the findings, confirm their reproducibility, and identify potential biases or flaws. Without such a publication, the claims about 'Bone 02's safety, efficacy in over 150 patients, and long-term outcomes remain unverified. It prevents other research teams from understanding the science, attempting to replicate the results, or building upon the innovation. The lack of a clinical trial registration number further compounds this issue, making it impossible for external parties to track the study's progress or access its detailed protocol and results.
Regulatory Hurdles
Even with transparent scientific data, the path from a successful clinical study to a globally available medical device is long and arduous. 'Bone 02' is reported to have applied for Chinese and international PCT patents, which is a crucial first step in protecting intellectual property. However, a patent is not a substitute for regulatory approval from bodies like the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA).
To gain approval, a new medical device must undergo a series of rigorous, multi-phase clinical trials designed to prove its safety and efficacy in a large and diverse patient population. The clinical study on over 150 patients, while a good start, is likely a pilot or early-phase trial. It would need to be followed by larger, multi-center, randomized controlled trials with long-term follow-up to meet the stringent standards of international regulatory bodies. The fact that a competing product, Tetranite™, is already in the FDA approval process indicates the significant time and financial investment required for this journey.
Broader Implications for Clinical Practice and Beyond
Transforming Surgical Procedures
If the claims of 'Bone 02' are scientifically validated, the technology would instigate a paradigm shift in orthopedic surgical workflow. The ability to fix complex, comminuted fractures in a matter of minutes through a minimally invasive injection would dramatically improve procedural efficiency. This would reduce the amount of time patients spend under anesthesia, lower the risk of intraoperative complications, and potentially allow some procedures to be performed in outpatient settings, reducing the overall cost of care.
The simplicity of application, which reportedly requires a "simple injection" rather than a suite of complex surgical tools, also has the potential to streamline training and standardize surgical outcomes. The "glue gun" innovation from South Korea, which allows for real-time adjustments and application, further demonstrates this trend toward more flexible, on-the-fly surgical solutions.
Applications in Diverse Environments
The rapid and simple application of 'Bone 02' also makes it uniquely suited for environments outside of a traditional hospital operating room. Its potential use in emergency medicine, trauma centers, and disaster relief scenarios is particularly noteworthy. In mass casualty events, where the immediate priority is rapid stabilization of injuries to save lives and limbs, a quick, injectable bone adhesive could be a transformative tool. It could provide a far more robust and immediate fixation than traditional field treatments like splinting, which are designed to prevent further movement but do not provide definitive fixation.
Economic and Societal Impact
The long-term economic and societal impact of a technology like 'Bone 02' could be substantial. By eliminating the need for a second surgery to remove implants, the adhesive would not only save patients from the associated pain and recovery time but would also lead to significant long-term cost savings for healthcare systems. Furthermore, by reducing the risks of infection and other complications, the technology could lower the rate of costly revision surgeries and improve a patient's long-term quality of life. This could lead to a faster return to work and daily activities, providing a broad economic benefit.
Conclusion & Strategic Recommendations
Based on a thorough analysis of the available information, 'Bone 02' embodies the promise of a new generation of bio-inspired biomaterials. It holds the potential to solve a century-old problem in orthopedic medicine by offering a rapid, bioabsorbable, and minimally invasive solution for fracture fixation. The reported clinical results, while extremely promising, must be viewed with the understanding that they are currently unverified by the global scientific community.
For 'Bone 02' to transition from a compelling news story to a globally adopted medical device, a clear strategic pathway is required. The following recommendations are provided for its future development and potential market adoption:
- Prioritize Peer-Reviewed Publication: The research team must publish their findings in a high-impact, international, peer-reviewed journal. This is the single most important step to establish scientific credibility and allow for the independent validation and replication of their results, which is a fundamental requirement for a scientific breakthrough.
- Conduct Rigorous Multi-Center Trials: To gain regulatory approval and widespread clinical adoption, the team must initiate larger, multi-center clinical trials. These trials should be transparently registered on public databases and adhere to stringent international standards for data collection and long-term patient follow-up.
- Explore Targeted Applications: Given the reported mechanical properties, 'Bone 02' may not be suitable for all types of fractures. It should be initially targeted for applications where its properties align with clinical needs, such as non-load-bearing comminuted fractures or fixation of joint fragments, before attempting to replace hardware in major long-bone fractures.
- Investigate Broader Use Cases: The unique advantages of speed and simplicity suggest that 'Bone 02' could be a transformative tool in emergency and field medicine. Specific trials should be conducted to evaluate its efficacy and safety in these specialized environments, fully leveraging the material's unique capabilities.
In summary, 'Bone 02' is a powerful illustration of the future of regenerative medicine and biomaterials. While its full potential remains to be scientifically validated, the innovation provides a clear vision for a future of orthopedic care that is faster, less invasive, and more aligned with the body's natural healing processess.
