In the final weeks of 2023, a quiet, however monumental revolution in medicinal drugs became officially sanctioned. Without the worldwide fanfare that marked the first vaccines or organ transplants, regulatory bodies inside the United Kingdom and the United States gave their approval to Casgevy, a therapy geared toward treating sickle-cell disease and beta-thalassemia. While the approval of any new drug is significant, this event marked a true paradigm shift, a watershed moment that will be studied by historians of technology for centuries to come. This is because Casgevy isn't a conventional tablet or injection that treats symptoms; it's miles the arena's first approved medicine primarily based on CRISPR gene-enhancing technology. This landmark choice has effectively transformed a Nobel Prize-winning laboratory approach—once the world of theoretical biology and science fiction—right into a living, breathing prescription capable of correcting genetic mistakes at their source. For the millions of humans globally harassed by inherited illnesses, this approval isn't always just a scientific breakthrough; it is the tangible sunrise of a new generation of medication, one wherein the very code of existence is not seen as an unchangeable destiny but as an editable text, promising the possibility of 1-time therapies for lifelong afflictions. The adventure from coming across an obscure bacterial protection mechanism to infusing corrected human cells back into an affected person has been breathtakingly speedy, and with this primary therapy now a clinical reality, the floodgates for genetic therapy have swung wide open.
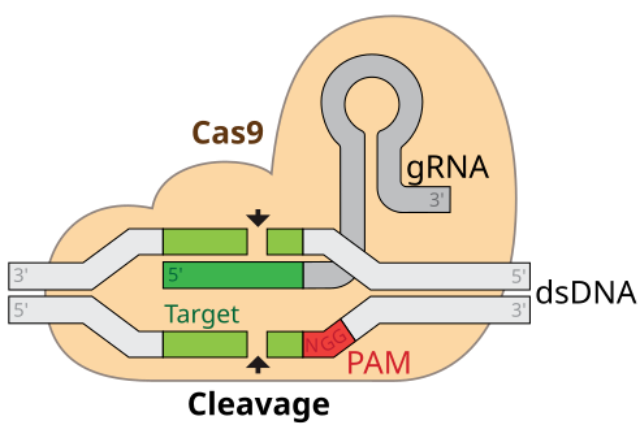
The technological know-how behind this medical miracle is both elegant and astonishingly unique, centered on the CRISPR-Cas9 machine, frequently described as a pair of "molecular scissors." This device includes key components: the Cas9 protein, which acts as the slicing tool, and a strand of guide RNA, which serves as a highly precise GPS, directing the scissors to an actual series inside someone's 3-billion-letter DNA code. For sufferers with sickle cell disorder, the healing procedure is a masterpiece of personalized medical treatment. Doctors start by harvesting the patient's very own hematopoietic stem cells—the very cells inside the bone marrow accountable for creating all blood cells. Outside the body, in a highly managed laboratory setting, those cells are edited using CRISPR. The molecular scissors are guided to a particular gene called BCL11A, which acts as a switch that deactivates the manufacturing of fetal hemoglobin rapidly after its start. By reducing this gene, the transfer is disabled, and the stem cells are reprogrammed to once more produce excessive tiers of fetal hemoglobin. This form of hemoglobin does not sickle and can correct the faulty person's hemoglobin that causes the disease. After this genetic amendment is complete, the affected person undergoes a path of chemotherapy to clean out their current, unedited bone marrow, making room for the corrected cells, which can then be infused again into their frame. These newly empowered stem cells take root and start a lifetime of producing healthy, spherical pink blood cells, functionally curing the patient of their disorder.
To simply draw close the significance of this development, one needs to comprehend the devastating fact of living with sickle-cell disease. Caused by a single-letter mutation in the gene for hemoglobin, the disease transforms bendy, disc-shaped red blood cells into inflexible, crescent or "sickle" shaped cells. These misshapen cells get stuck in small blood vessels, leading to a cascade of debilitating fitness issues. The maximum prominent symptom is excruciating, unpredictable ache crises that are experienced like sharp, stabbing glass transferring through the frame, frequently requiring hospitalization and heavy opioid painkillers. Beyond the pain, the disorder causes continual anemia, fatigue, an increased danger of stroke, and progressive damage to crucial organs like the lungs, kidneys, and heart, leading to a drastically shortened lifespan. It is a relentless, all-consuming situation that dictates every issue of someone's lifestyle, from their career alternatives to their own family planning. The approval of a CRISPR-based therapy offers more than simply the absence of signs; it offers the return of a life. For the teenagers in clinical trials who have long gone from month-to-month sanatorium visits to strolling marathons and planning futures they never thought possible, this one-time remedy represents a liberation from a genetic jail, a chance to live without consistent ache and worry, and to redefine themselves beyond the confines of their analysis.
However, the sunrise of this genetic revolution isn't always without its substantial shadows and ambitious, demanding situations. The most instant and obvious hurdle is the wonderful rate tag. With a cost of over two million dollars for each affected person, this life-altering remedy is currently accessible only to a tiny fraction of individuals who need it, raising profound questions about equity and the commercialization of treatment plans. The vast majority of human beings with sickle-cell disease live in lower-income countries, particularly in sub-Saharan Africa, where one of these diseases is an insurmountable challenge, creating a new and tragic frontier of healthcare disparity. Furthermore, the remedy procedure itself is an arduous and physically annoying ordeal. The required chemotherapy to wipe out the existing bone marrow carries its own dangers, including infertility and the risk for secondary cancers. While the precision of CRISPR is splendid, issues over long-term protection persist, in particular the danger of "off-target" edits, wherein the molecular scissors inadvertently reduce the DNA at an unintended area, which could have unknown consequences. These troubles of value, access, complexity, and long-term protection ensure that, at the same time as step one has been taken, the direction to making genetic remedies a standard, worldwide answer will be lengthy, complex, and fraught with ethical and logistical dilemmas that society is just starting to confront.
Despite these hurdles, the approval of the first CRISPR-based medicine is an inflection point for humanity. It has irrevocably unlocked the door to a destiny where we will address disease at its maximum essential level. The achievement with sickle cell disease serves as a powerful evidence-of-concept, and research is already properly underway to apply the identical era to a bunch of other genetic issues, from muscular dystrophy and cystic fibrosis to Huntington's disorder and inherited forms of blindness. The capability applications increase even beyond uncommon genetic sicknesses, with scientists exploring CRISPR-based edits to strengthen immune cells to fight cancer, lessen dangerously excessive levels of cholesterol, or even confer resistance to viruses like HIV. This fast progress unavoidably forces a deeper, more pressing communication about the moral limitations of this electricity. While enhancing the somatic (non-reproductive) cells of a sick patient is widely celebrated, the opportunity of germline modifying—making heritable adjustments to sperm, eggs, or embryos—remains an extraordinarily contentious ethical minefield. We now stand at the start of a new chapter in the human story, one where we are transitioning from being mere readers of the genetic code to its authors. The obligation that comes with this energy is significant, but the promise it holds for alleviating human suffering is certainly modern.
