The Indian Pitcher Plant Nepenthes

Source: pin.it
Carnivorous Plants:
There are thought to be a little over 600 species of carnivorous plants, the bulk of which are found in the Orders Caryophyllales and Lamiales. The monotypic Nepenthaceae (Caryophyllales), the largest Family of pitcher plants, has over 100 species. Nepenthes pitcher plants are only found in the Palaeotropics, from Madagascar to New Caledonia and a few distant western Pacific islands in the east. The Greater Sunda islands of Borneo and Sumatra, the Philippines, Sulawesi, and the Indonesian archipelago as a whole are exceptionally species-rich.
The most peculiar adaptations to low-nutrient settings are found in carnivorous plants. These plants capture and devour a variety of insects, occasionally even tiny frogs and animals, to gain some nutrients. They are sometimes referred to as insectivorous plants because insects are one of the most popular prey items for the majority of carnivorous plants. It is not unexpected that bogs and fens, where nutrient concentrations are low yet water and sunlight are abundant during certain seasons, are the most frequent home for these plants. A single bog has up to thirteen different species of carnivorous plants (Folkerts, 1982). The majority of plants use their roots to take nitrogen from the soil. Carnivorous plants, however, use their particularly modified leaves as traps to extract nitrogen from their animal food.
Tropical pitcher plant- Nepenthes
There are more than 200 species of nepenthes, which are enormous climbing carnivorous plants. They develop into enormous sizes and hang as vines with enormous pitchers. Although you can grow tropical pitcher plants practically anywhere in a greenhouse or terrarium, they are best suited for warm or hot climates.
Because monkeys utilise them to drink water, tropical pitcher plants are often known as "Monkey Cups" (not true). Nepenthes are a worldwide plant, however they are most prevalent in Southeastern Asia. Indonesia, the Philippines, Sumatra, New Caledonia, China, Borneo, and Australia are among the nations that are home to tropical pitcher plants.
Nepenthes come in both male and female varieties, with the majority of the plants being male.

Source: static.wixstatic.com
Traps of Nepenthes:
Pitfall traps on nepenthes are stuffed with digestive fluids. The pitfall traps take months to mature after beginning as a leaf. Although the trap does not move in order to catch the prey, it is huge and many different insects, including ants and wasps, fall prey to this plant.Because the plant produces nectar, insects are drawn to it. Many insects go up to the pitcher to sample the nectar before stepping into the trap.
Life of Nepenthes:
Tropical Pitcher plants live for many years, often ranging from 10-20 years. The plant can take 5-10 years to flower and it will have new shoots growing each year that turn into rosettes.
After the plant has flowered, it will continue growing stems. This way, the plant will always continue growing throughout its life.
Prey Attraction
The inner surfaces of nepenthes pitchers exhibit a clear functional zonation and are gravity-driven passive traps. The locations of the highest density of extrafloral nectaries are the pitcher lid and peristome (a collar-shaped structure enclosing and overhanging the mouth). It has been shown that some species' aerial pitchers emit smell, which draws anthophilous (flower-visiting) insects. In some animals, colour patterns may also be used to draw in prey. For instance, the peristomes of N. Rafflesiana pitchers (with wavelengths between 350 and 370, 430 and 470, and 490-540 nm, respectively) are quite conspicuous in comparison to the pitcher body. The pitchers are very noticeable to many anthophilous taxa because these locations correlate to insect visual sensitivity maxima. A potent example of convergent evolution is the presence of nectar, scent, and visual orientation signals to a nectar source in Nepenthes pitchers, which are considered to be floral in origin. Both Nepenthes pitchers and insect-pollinated blooms have developed to attract and hold insects at the location where the plant will gain most from doing so, first through the transfer of gametes and second through the acquisition of limited nutrients.

Source: www.etsy.com
Not all Nepenthes species provide visitors only nectar as a reward. Nepenthes albomarginata T. Lobb ex Lindl. pitchers generate relatively little nectar in contrast to pitchers of other species. On the outside of the pitcher, just below the peristome, there is a noticeable band of tomentose tissue that is cream in colour; this is where the species gets its name. Hospitalitermes (Isoptera) termites, which forage in columns with tens of thousands of workers, are attracted to this tissue and feed on lichen. It has been roughly estimated that termites contribute for >50% of the foliar nitrogen (N) in this species because they are frequently trapped in considerable numbers by N. albomarginata pitchers.
Prey Capture and Retention
The peristome, which has a high nectary density as mentioned above, is a crucial part of the prey attraction system. The function of the peristome in the actual mechanics of prey capture has been determined by a number of clever investigations. Rows of overlapping epidermal cells arranged in radial ridges cover the peristome's surface. This configuration has two benefits that aid in capturing prey.
- The first is anisotropy, in which all of the epidermal cells overlap one another starting from the peristome's outside edge inward. Therefore, even though it is relatively simple for an insect to establish traction with its claws while moving towards the pitcher mouth (and thus capture), it struggles to hold on while moving in the pitcher.
- The second property is capillary action-based wettability. The nectar produced at the inner rim of the peristome itself or moisture in the environment can both wet the peristome, making it extremely slippery and causing invertebrates to lose their grip and tumble into the pitcher mouth. However, given the near universality of the usual peristome structure across Nepenthes, it is likely that the mechanisms described above are common throughout the genus. The research was done on N. rafflesiana and Nepenthes bicalcarata Hook.
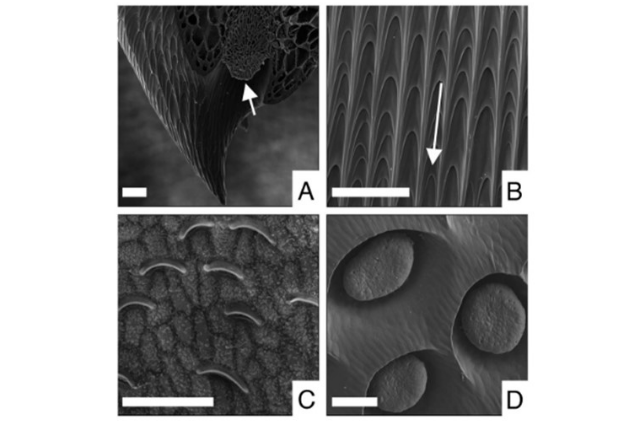
The prey comes across additional elements that aid its downward trajectory once it has escaped the peristome and entered the pitcher itself. Depending on the species, the inner pitcher surface's upper part may or may not have a significant number of lunate cells. These are modified stomatal guard cells that are angled to dangle over the pitcher wall, preventing invertebrate claws from gaining traction. The existence of layered epicuticular waxes is the second characteristic of this zone, which varies depending on the species. Despite possible differences in chemical makeup between species, all waxes contain primary alcohols and very long-chain aldehydes.
The waxy zone prevents traction in two ways: first, the claws' efficiency is decreased by the outer layer's wax crystals clogging them, and second, the wax surface has a low free surface energy. As a result, the inner pitcher wall is rendered impervious to moisture, preventing insects with hairy pulvillae from acquiring traction through capillary action. The prey will eventually fall into the liquid that gathers in the pitcher's lower portion. This highly viscoelastic fluid of N. rafflesiana keeps the prey firmly in place until it drowns by preventing it from freeing itself. According to a recent study, the viscoelasticity of the pitcher fluid may be more important for this species' retention of prey than lunate cells or epicuticular waxes.
The fluid produced by pitchers of Nepenthes inermis Danser, Nepenthes aristolochioides Jebb & Cheek, Nepenthes jacquelinae Clarke, Davis & Tamin, Nepenthes dubia Danser, and Nepenthes talangensis Nerz & Wistuba has similar qualities to that of N. rafflesiana.
Digestion of Prey and Uptake of Nutrients
In addition to drowning the prey, pitcher fluid permits enzymatic breakdown of the carcasses and ingestion of the digestive byproducts. Numerous digestive glands are present in the lower portion of the inner pitcher wall and perform two ontogenetically predetermined tasks. The glands release the watery digesting fluid in juvenile pitchers. Numerous enzymes, including proteases, peptidases, phosphatases, esterases, ribonucleases, and chitinases, have been found to be present in this. Free radicals and a protein called TLP with antibacterial and antifungal characteristics may also be produced by the glands to aid in the breakdown of prey tissue. The glands convert from secreting enzymes to absorbing the byproducts of enzymatic breakdown, which take the form of amino acids, peptides, and ammonium ions, once the pitcher has reached maturity. Proton pumps work to maintain the pH that is ideal for enzyme activity.
Costs and Benefits of Carnivory
In their natural environments, Nepenthes are N-limited, just like other carnivorous plants. It has been noted for various taxa how much additional nutrients may be obtained through carnivory. The Nepenthaceae still need to be studied, though. The plant incurs enormous expenses as a result of the resources needed to produce pitchers. A functional trade-off that ultimately lowers photosynthetic efficiency is the creation of a leaf that serves both as a carnivore and a light harvester. A substantial evolutionary drive to supplement or even replace root-mediated nutrition with animal-derived inputs is implied by Nepenthes' use of sophisticated and expensive trapping structures, and research to date supports this hypothesis.
In Nepenthes mirabilis Druce, for instance, there is an ontogenic switch from mostly root-derived N absorption to primarily pitcher-derived N uptake via prey inputs as the plant matures. Formicidae capture is estimated to supply up to 68 percent of foliar N in N. rafflesiana. A starvation study in which N. rafflesiana plants were denied prey inputs revealed increased foliar reflectance in the photosynthetically-active waveband (608-738 nm) compared to control plants, signifying degradation of photosynthetic capacity. Prey capture confers physiological benefits by alleviating N deficits. In comparison to controls, the impacts of this loss were evident in a considerable decline in the pace of pitcher production as well as a drop in pitcher size. Similarly, it was discovered that feeding N. Talangensis insect prey dramatically increased photosynthetic efficiency.
Conclusion
This brief summary makes clear that over the past few decades, experts from a variety of fields have clarified a number of important features of the carnivorous sickness in Nepenthes. Many of the findings reported here have raised new concerns, which is maybe equally significant. The pathways listed below are just a few that we believe could pay off future research efforts; they are by no means meant to be an exclusive list.
The mechanism of attraction:
Despite reports that N. rafflesiana uses scent to lure prey, the volatile chemicals involved have not been identified. It has to be seen to what extent the pitchers of other Nepenthes species may utilise fragrance, as has lately been done for other carnivorous plant genera. Only a relatively small number of species have had their use of colour patterns to guide prey toward the nectar source studied. It could also be worthwhile to look into the extent to which nectar composition (such as sugar composition/concentration, amino acids, etc.) may reflect the demands of particular prey, similar to how many angiosperms tailor their nectar to the requirements of their pollinators.

